Conformational and mechanistic studies in sugars
- Sugar amino acids - monomers and dimers
Sugar amino acids, SAA, are derivatives of saccharides that have an amino and carboxyl group attached to the sugar ring. Compounds of this type have been used as glycomimetics, peptidomimetics, as well as isosteres, platforms with pharmacological recognition points and foldamers. SAAs are a structurally very diverse group of sugar derivatives. This diversity concerns both the size of the ring, the distribution of functional groups, the presence of other groups in the ring, the configuration of the substituents attached to the ring and conformation. Such diversity provides a huge number of possible combinations.
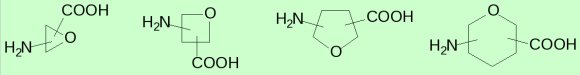
The research goal is to describe the conformational space of mono- and dimers of sugar amino acids.
- Quaternary ammonium salts - conformational aspects of the furanoid ring and formation
Quaternary ammonium salts (CSA) containing sugar residues are the subject of many years of research at the Sugar Chemistry Group. In the course of these studies, a relationship between the structure of tosylate derivatives of sugars and anhydroalditols and the rate of their quaternization under the influence of various types of tertiary amine was found. These observations became the starting point for computational research, the purpose of which was to confirm the observations made, as well as to indicate the factors influencing the course of the tested reactions. Another aspect of research on the formation of CSA is the description of the conformational behavior of the furanoid ring during the reaction.
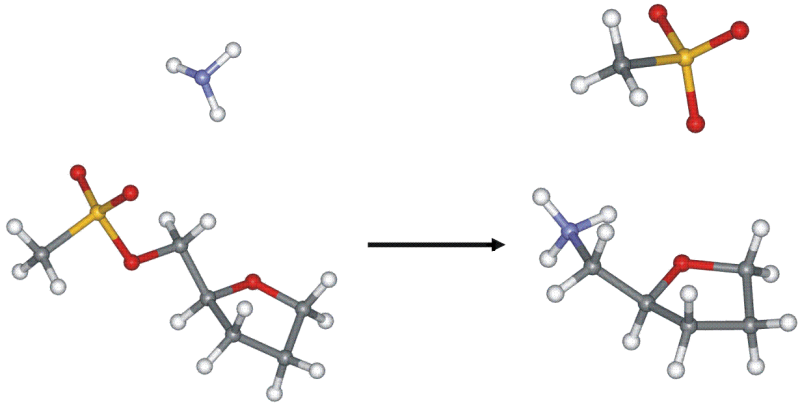
Publications:
- Nowacki, A., Liberek, B., Comparative conformational studies of 3,4,6-tri-O-acetyl-1,5-anhydro-2- deoxyhex-1-enitols at the DFT level, Carbohydr. Res. 2018, 462, 13-27
- Walczak, D., Nowacki, A., Trzybiński, D., Samaszko-Fiertek, J., Myszka, H., Sikorski, A., Liberek B., Conformational studies of N-(α-D-glucofuranurono-6,3-lactone)- and N-(methyl β-D-glucopyranuronate)-p-nitroanilines, Carbohydr. Res., 2017, 446-447, 85-92
- Cyman, M., Wielińska, J., Myszka, M., Trzybiński, D., Sikorski, A., Nowacki, A., Liberek B., Influence of the oxime and anomeric configurations on the stability of 2-deoxy-2-hydroxyimino-D-hexopyranosides J. Mol. Struct. 2016, 1125, 558-569
- Bednarko, J., Wielińska, J., Sikora, K., Liberek, B., Nowacki A., Theoretical studies on the reaction of mono- and ditriflate derivatives of 1,4:3,6-dianhydro-D-mannitol with trimethylamine – Can a quaternary ammonium salt be a source of the methyl group? J. Comput. Aided Mol. Des. 2016, 30, 13-26
- Wielińska, J., Liberek, B., Nowacki, A. DFT studies of the formation of furanoid derivatives ofammonium chlorides, J. Mol. Graph. Model. 2015, 56, 74–83
- Nowacki, A., Wielińska, J., Walczak. D., Sikora, K., Dmochowska, B., Liberek, B. The conformational behavior, geometry and energy parameters of Menshutkin-like reaction of O-isopropylidene-protected glycofuranoid mesylates in view of DFT calculations, J. Mol. Graph. Model. 2014, 52, 91–102
- Walczak, D., Nowacki, A. DFT studies of conversion of methyl chloride and three substituted chloromethyl tetrahydrofuran derivatives during reaction with trimethylamine J Mol Model, 2013, 19, 4403-4417
- Nowacki, A., Myszka, H., Liberek, B. Conformational studies of diosgenyl 2-amino-2-deoxy-β-D-glucopyranosides at the PM3 and DFT levels of theory Carbohydr. Res., 2013, 377, 4-13
- Nowacki, A., Sikora, K., Dmochowska, B., Wiśniewski, A. DFT studies of the conversion of four mesylate esters during reaction with ammonia J. Mol. Mod., 2013, 19, 3015-3026
- Nowacki, A., Liberek, B. Acetylated methyl 1,2-dideoxyhex-1-enopyranuronates in density functional theory conformational studies Carbohydr. Res., 2013, 371:1-7
- Nowacki, A., Sikora, K., Dmochowska, B., Wiśniewski, A. Studies of the formation of N-substituted pyridinium mesylates: A theoretical approach Comp. Theor. Chem., 2012, 1000:33-41
- Nowacki, A., Dmochowska, B., Sikora, K., Madaj, J., Wiśniewski, A. Theoretical studies of the formation of quaternary pyridinium mesylates Comput. Theoret. Chem., 2012, 986:85-92
- Nowacki, A., Walczak, D., Liberek, B. Fully acetylated 1,5-anhydro-2-deoxypent-1-enitols and 1,5-anhydro-2,6-dideoxyhex-1-enitols in DFT level theory conformational studies Carbohydr. Res., 2012, 352:177-185
- Nowacki, A., Dmochowska, B., Jączkowska, E., Sikora, K., Wiśniewski, A. Theoretical studies of the formation of quaternary ammonium mesylates Comput. Theoret. Chem., 2011, 973:53-61
- Nowacki, A., Liberek, B. Methyl 3-Amino-2,3,6-trideoxy-L-hexopyranosides in DFT Level Theory Conformational Studies J. Phys. Chem. A, 2008, 112:7072-7079
- Liberek, B., Nowacki, A. Methyl 4-O-Acetyl-3-azido- and 3-Azido-4-O-methylsulfonyl-2,3,6,-trideoxyhex-5-eno Pyranosides in DFT-Level Conformational Studies J. Phys. Chem. A., 2007, 111:4397-4403




